Name
Virtual Chemistry
Unit 7 HW 4
Answer questions 1-12 about the phase diagram shown...

Chemistry, 12.02.2021 18:30 gabriellabadon2
Name
Virtual Chemistry
Unit 7 HW 4
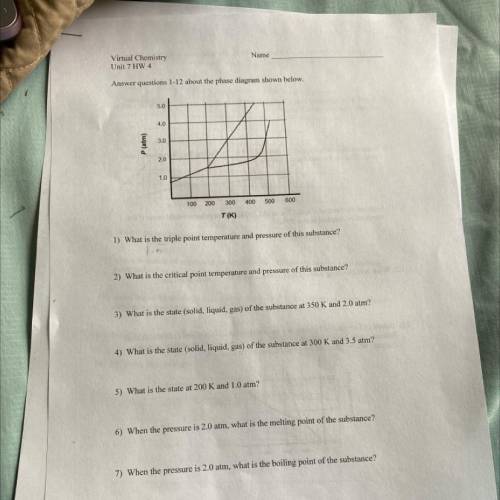
Answer questions 1-12 about the phase diagram shown below.
5.0
4.0
3.0
P (atm)
2.0
1.0
100 200
300
400
500
600
T(K)
1) What is the triple point temperature and pressure of this substance?
2) What is the critical point temperature and pressure of this substance?
3) What is the state (solid, liquid, gas) of the substance at 350 K and 2.0 atm?
4) What is the state (solid, liquid, gas) of the substance at 300 K and 3.5 atm?
5) What is the state at 200 K and 1.0 atm?
6) When the pressure is 2.0 atm, what is the melting point of the substance?
7) When the pressure is 2.0 atm, what is the boiling point of the substance?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Questions

Mathematics, 17.06.2020 22:57






English, 17.06.2020 22:57

Biology, 17.06.2020 22:57

Mathematics, 17.06.2020 22:57



Mathematics, 17.06.2020 22:57



Mathematics, 17.06.2020 22:57

Mathematics, 17.06.2020 22:57






