
Chemistry, 19.09.2019 18:30 woodfordmaliky
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(aq)+3h2(g) (deltah)= -725.0 kj
2. hcl(> hcl(aq) (deltah)= -74.8kj 3. h2(g)+cl2(g) --> 2hcl(g) (deltah)=-1845.0kj 4. mcl3(s) --> mcl3(aq) (deltah)= -476.0kj use the information above to determine the enthalpy of the following reaction.
2m(s)+3cl2(g) > 2mcl3(s) (deltah) = kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
Questions

History, 20.10.2019 05:50



Health, 20.10.2019 05:50


Mathematics, 20.10.2019 05:50

History, 20.10.2019 05:50

Advanced Placement (AP), 20.10.2019 05:50

Biology, 20.10.2019 05:50


Social Studies, 20.10.2019 05:50

Mathematics, 20.10.2019 05:50


Mathematics, 20.10.2019 05:50


Mathematics, 20.10.2019 05:50




Mathematics, 20.10.2019 05:50

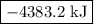
 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.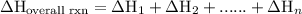
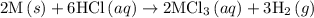 ...… (1)
...… (1)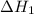 is -725 kJ.
is -725 kJ.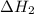 .
.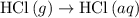 ...... (2)
...... (2) 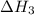 .
.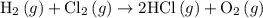 …… (3)
…… (3)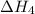 .
.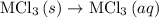 …… (4)
…… (4)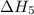 .
.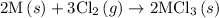 …… (5)
…… (5)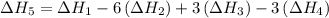 ...... (7)
...... (7) 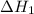 , -74.8 kJ for
, -74.8 kJ for 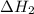 and -1845 kJ for
and -1845 kJ for 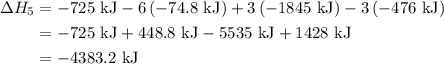
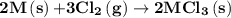 is -4383.2 kJ.
is -4383.2 kJ.

