
Chemistry, 12.02.2021 02:10 sdfghyuji123
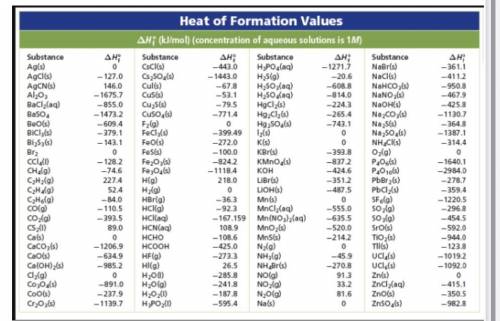
Use Hess’s Law and solve for the enthalpy change of the following rxn:
12 P(s) + 10 Fe2O3 (s) -> 3 P4O10 (s) + 20 Fe (s)
2 C2H6 (l) + 7 O2 (g) -> 4 CO2 (g) + 6H2O (g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

You know the right answer?
Use Hess’s Law and solve for the enthalpy change of the following rxn:
12 P(s) + 10 Fe2O3 (s) ->...
Questions




Computers and Technology, 27.08.2019 19:40

History, 27.08.2019 19:40

Mathematics, 27.08.2019 19:40

Physics, 27.08.2019 19:40

Mathematics, 27.08.2019 19:40

History, 27.08.2019 19:40

English, 27.08.2019 19:40


Mathematics, 27.08.2019 19:40

Social Studies, 27.08.2019 19:40



Mathematics, 27.08.2019 19:40

Biology, 27.08.2019 19:40



Social Studies, 27.08.2019 19:40



