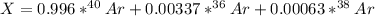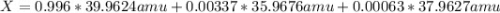Argon has three naturally-occurring isotopes: 99.6% of 40Ar, with an atomic weight of 39.9624 amu, 0.337% of 36Ar, with an atomic weight of 35.9676 amu, and 0.063% of 38Ar, with an atomic weight of 37.9627 amu. Calculate the average atomic weight of Ar. Round off the answer to six significant figures. Do not include the units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Argon has three naturally-occurring isotopes: 99.6% of 40Ar, with an atomic weight of 39.9624 amu, 0...
Questions



Health, 16.09.2019 15:10

English, 16.09.2019 15:10




Biology, 16.09.2019 15:10

Physics, 16.09.2019 15:10

Mathematics, 16.09.2019 15:10

Mathematics, 16.09.2019 15:10

Spanish, 16.09.2019 15:10

Physics, 16.09.2019 15:10

Chemistry, 16.09.2019 15:10


History, 16.09.2019 15:10

Computers and Technology, 16.09.2019 15:10


Biology, 16.09.2019 15:10

Physics, 16.09.2019 15:10