Chemistry, 11.02.2021 22:20 angelinagiraffp538zb
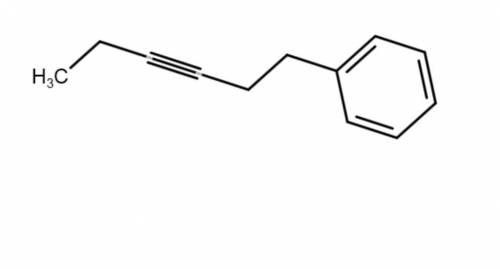
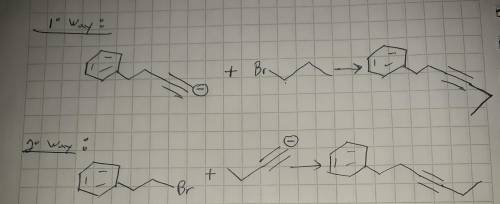
This molecule can be synthesized from an alkyne anion and an alkyl bromide. However, there are two ways in which this molecule can be formed. One way uses a higher molecular weight alkyne anion (Part 1) and the other uses a lower molecular weight anion (Part 2). Draw the two versions in the boxes below. Omit spectator ions.
For Part 1: Draw the reactants (i. e., alkyne anion and alkyl bromide) needed for the pathway that uses a higher molecular weight alkyne anion:
For Part 2: Draw the reactants (i. e., alkyne anion and alkyl bromide) needed for the pathway that uses a lower molecular weight alkyne anion:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
This molecule can be synthesized from an alkyne anion and an alkyl bromide. However, there are two w...
Questions


Physics, 26.06.2019 02:00


Physics, 26.06.2019 02:00


Health, 26.06.2019 02:00




Business, 26.06.2019 02:00





Mathematics, 26.06.2019 02:00



Chemistry, 26.06.2019 02:00

Mathematics, 26.06.2019 02:00

History, 26.06.2019 02:00