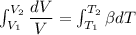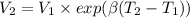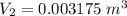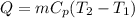Chemistry, 11.02.2021 21:20 st23pgardner
Five kilograms of liquid carbon tetrachloride undergo a mechanically reversible, isobaric change of state at 1 bar during which the temperature changes from 0∘C to 20∘C0 ∘ C to 20 ∘ C Determine ΔVt, W,Q,ΔHt, and ΔUt.ΔV t ,W, Q,ΔH t , and ΔU t . The properties for liquid carbon tetrachloride at 1 bar and 0∘C0 ∘ C may be assumed independent of temperature: β=1.2×10−3K−1,CP=0.84kJ⋅kg−1⋅K−1, and rho=1590kg⋅m−3β=1.2×10 −3 K −1 ,C P =0.84kJ⋅kg −1 ⋅K −1 , and rho=1590kg⋅m −3

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Five kilograms of liquid carbon tetrachloride undergo a mechanically reversible, isobaric change of...
Questions

Mathematics, 28.10.2020 06:40

Mathematics, 28.10.2020 06:40

Mathematics, 28.10.2020 06:40


History, 28.10.2020 06:40

Mathematics, 28.10.2020 06:40

Mathematics, 28.10.2020 06:40


Physics, 28.10.2020 06:40

History, 28.10.2020 06:40


Mathematics, 28.10.2020 06:40

Computers and Technology, 28.10.2020 06:40

Physics, 28.10.2020 06:40

History, 28.10.2020 06:40

Mathematics, 28.10.2020 06:40


Mathematics, 28.10.2020 06:40



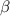 is independent of temperature while pressure is constant;
is independent of temperature while pressure is constant;