
Chemistry, 11.02.2021 18:30 jcastronakaya
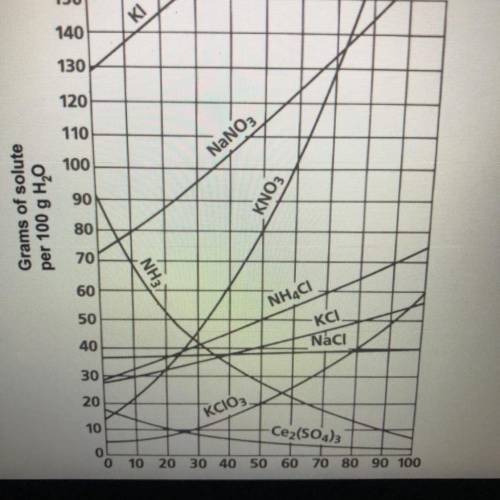
Continue to use the solubility curve graph to determine if the following solutions are
saturated or unsaturated. If they are unsaturated determine how much more solute
should be added to make a saturated solution.
7. (10 Points) A solution that contains 70g of NaNO3 at 30°C (in 100 mL H2O)
8. (10 Points) A solution that contains 50g of NH, Cl at 50°C (in 100 mL H,0)
9. (10 Points) A solution that contains 70g of KI at 0°C (in 100 mL H,0)
10. (10 Points) A solution that contains 20g of KCloz at 50°C (in 100 mL H2O)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
Continue to use the solubility curve graph to determine if the following solutions are
saturated or...
Questions

Mathematics, 18.03.2021 07:10


Chemistry, 18.03.2021 07:10



Chemistry, 18.03.2021 07:10


Mathematics, 18.03.2021 07:10


Mathematics, 18.03.2021 07:10


Mathematics, 18.03.2021 07:10

Mathematics, 18.03.2021 07:10

Mathematics, 18.03.2021 07:10



Mathematics, 18.03.2021 07:10

Mathematics, 18.03.2021 07:10

Mathematics, 18.03.2021 07:10

Spanish, 18.03.2021 07:10



