
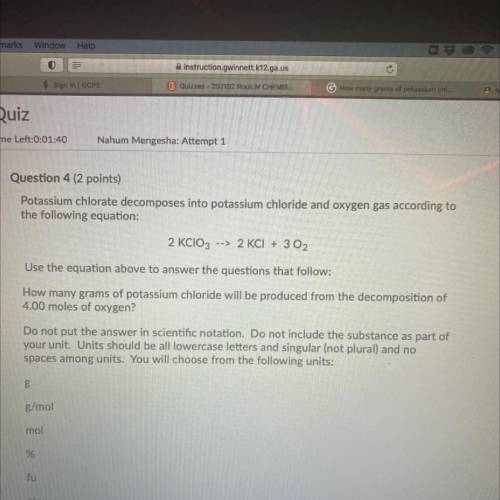
Question 4 (2 points)
Potassium chlorate decomposes into potassium chloride and oxygen gas according to
the following equation:
2 KClO3 --> 2 KCl + 3 O2
Use the equation above to answer the questions that follow:
How many grams of potassium chloride will be produced from the decomposition of
4.00 moles of oxygen?
Do not put the answer in scientific notation. Do not include the substance as part of
your unit. Units should be all lowercase letters and singular (not plural) and no
spaces among units. You will choose from the following units:
В
g/mol
mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 23.06.2019 21:30
Which particles make up the nucleus of an atom? a. protons and electrons b. neutrons and electrons c. protons only d. protons and neutrons e. neutrons only
Answers: 1

Chemistry, 23.06.2019 21:50
Agas engine that operates on a brayton cycle has an efficiency of 0.23. on a cold day, the temperature of the air drawn into the engine is 267 k.part awhat is the temperature of the air exhausted from the engine?
Answers: 3

Chemistry, 23.06.2019 22:00
The following compounds, listed with their boiling points, are liquid at –10ºc: butane, –0.5ºc; ethanol, 78.3ºc; toluene, 110.6ºc. at –10ºc, which of these liquids would you expect to have the highest vapor pressure? which the lowest? explain. (4 points)
Answers: 1
You know the right answer?
Question 4 (2 points)
Potassium chlorate decomposes into potassium chloride and oxygen gas accordin...
Questions

Mathematics, 22.07.2019 09:00



Social Studies, 22.07.2019 09:00






English, 22.07.2019 09:00

History, 22.07.2019 09:00


History, 22.07.2019 09:00

Biology, 22.07.2019 09:00

Social Studies, 22.07.2019 09:00







