
Chemistry, 11.02.2021 02:10 blessednish86orogbi
Answer questions 1-6 using the information below:
2 A(aq) + 3 B(aq) LaTeX: \longrightarrow⟶ A2B3(aq)
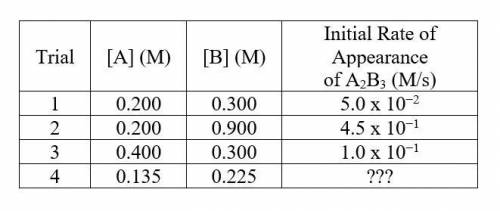
An experiment is conducted at 25 C and the rate of appearance of the product A2B3(aq) is measured as the concentrations of the reactants were varied. Data from the trials are shown below: -picture-
1. Determine the rate law for the reaction at 25 C. Justify your answer.
2. Determine the value of k, including units.
3. Determine the rate of trial 4.
4. A possible reaction mechanism has a 1st elementary step as shown below:
A + B ⟶ AB
Could this first step be the rate determining step? Explain your reasoning.
5. In the reaction mechanism, the compound MnO2 appears. A student makes the claim:
"The order of MnO2 must be zero since it does not appear in the overall balanced equation."
Do you agree or disagree with the student? Explain your reasoning.
6. In trial 1, 10 mL of A and 10 mL of B are mixed and the reaction goes to completion.
i.) Determine the number of moles of B used in the reaction.
ii.) The reaction vessel is heated and all the water is driven off, leaving only 0.124 grams of A2B3(s). Determine the molar mass of A2B3.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Answer questions 1-6 using the information below:
2 A(aq) + 3 B(aq) LaTeX: \longrightarrow⟶ A2B3(aq...
Questions

Business, 10.03.2021 04:20

History, 10.03.2021 04:20

Spanish, 10.03.2021 04:20

Computers and Technology, 10.03.2021 04:20





Mathematics, 10.03.2021 04:20



Mathematics, 10.03.2021 04:20

Mathematics, 10.03.2021 04:20

Social Studies, 10.03.2021 04:20

History, 10.03.2021 04:20

English, 10.03.2021 04:20

Arts, 10.03.2021 04:20

Chemistry, 10.03.2021 04:20

English, 10.03.2021 04:20



