
Chemistry, 10.02.2021 22:10 kiannadgarnica
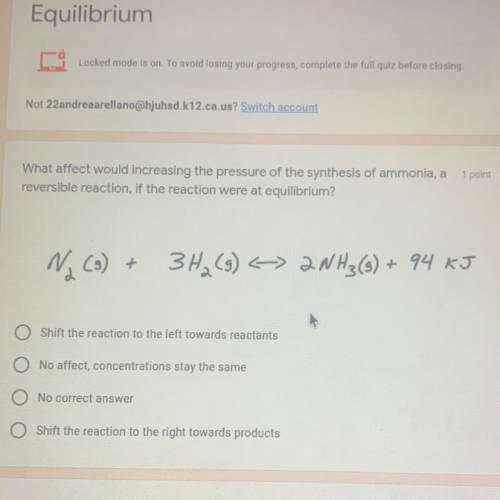
What affect would increasing the pressure of the synthesis of ammonia, a
reversible reaction, if the reaction were at equilibrium?
Na (3) +
3H₂(g) < 2NH3(s) + 94 KJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
What affect would increasing the pressure of the synthesis of ammonia, a
reversible reaction, if th...
Questions


English, 06.12.2020 07:10

Mathematics, 06.12.2020 07:10

Health, 06.12.2020 07:10


Social Studies, 06.12.2020 07:10



Advanced Placement (AP), 06.12.2020 07:10

Mathematics, 06.12.2020 07:10


Mathematics, 06.12.2020 07:10

Social Studies, 06.12.2020 07:10

Mathematics, 06.12.2020 07:10

Mathematics, 06.12.2020 07:10

Mathematics, 06.12.2020 07:10

English, 06.12.2020 07:10


Chemistry, 06.12.2020 07:10



