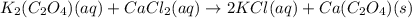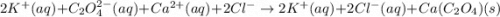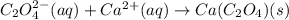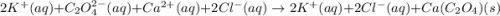Chemistry, 10.02.2021 14:00 gaceves6177
Write a balanced molecular, ionic, and net ionic equation for the following reaction. Assume the reaction occurs in
aqueous solution.
K2(C2O4)(aq)+CaCl2(aq) to
2KCl(aq)+ Ca(C2O4)(s)
balanced?
Ionic Equation:
Net Ionic Equation:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1


Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
Write a balanced molecular, ionic, and net ionic equation for the following reaction. Assume the rea...
Questions


Mathematics, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01

English, 22.10.2020 18:01



Social Studies, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01



Chemistry, 22.10.2020 18:01





Computers and Technology, 22.10.2020 18:01




History, 22.10.2020 18:01