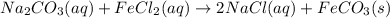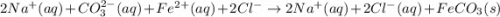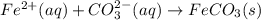Chemistry, 10.02.2021 14:00 bryanmcmillianjr
Write a balanced molecular, ionic, and net ionic equation for the following reaction. Assume the reaction occurs in
aqueous solution.
Na2Co3(aq)+FeCl,(aq) to
FeCo3(s) + NaCl(aq)
balanced:
Ionic Equation:
Net Ionic Equation:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

You know the right answer?
Write a balanced molecular, ionic, and net ionic equation for the following reaction. Assume the rea...
Questions



Biology, 21.07.2019 14:30

History, 21.07.2019 14:30

History, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30


Biology, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30


Biology, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30


Social Studies, 21.07.2019 14:30


Physics, 21.07.2019 14:30