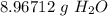Chemistry, 10.02.2021 06:00 witchhunt666
What is the mass of 3.00x10^23 particles of water? Water has a molar mass of 18.0 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
You know the right answer?
What is the mass of 3.00x10^23 particles of water? Water has a molar mass of 18.0 g/mol....
Questions

Social Studies, 27.08.2019 03:40



Computers and Technology, 27.08.2019 03:40




Computers and Technology, 27.08.2019 03:40


English, 27.08.2019 03:40


Geography, 27.08.2019 03:40


Mathematics, 27.08.2019 03:40



Mathematics, 27.08.2019 03:40



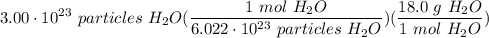 Multiply/Divide:
Multiply/Divide: