Hello i would realy appreciate if anyone can solve this one step question :))!!!
...

Chemistry, 10.02.2021 05:20 Alinakohshsb
Hello i would realy appreciate if anyone can solve this one step question :))!!!
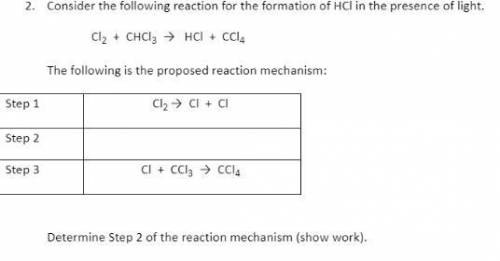

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Questions



Mathematics, 18.07.2019 12:00


Social Studies, 18.07.2019 12:00


Biology, 18.07.2019 12:00



Arts, 18.07.2019 12:00

Arts, 18.07.2019 12:00


English, 18.07.2019 12:00







World Languages, 18.07.2019 12:00



