
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganate. They react according to the following equation
KMnO4 + K2C2O4 → K2Mn + 2CO2 + 202
If the reaction has a 53% yield, how many grams of carbon dioxide does it produce?
А
11.79
B
44.09
23.39
2209
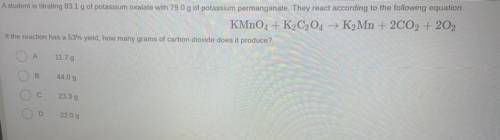

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganat...
Questions

Mathematics, 23.12.2019 07:31

Mathematics, 23.12.2019 07:31



Chemistry, 23.12.2019 07:31

Mathematics, 23.12.2019 07:31


Mathematics, 23.12.2019 07:31

History, 23.12.2019 07:31

Mathematics, 23.12.2019 07:31



Mathematics, 23.12.2019 07:31





Geography, 23.12.2019 07:31

Mathematics, 23.12.2019 07:31



