Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1


Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
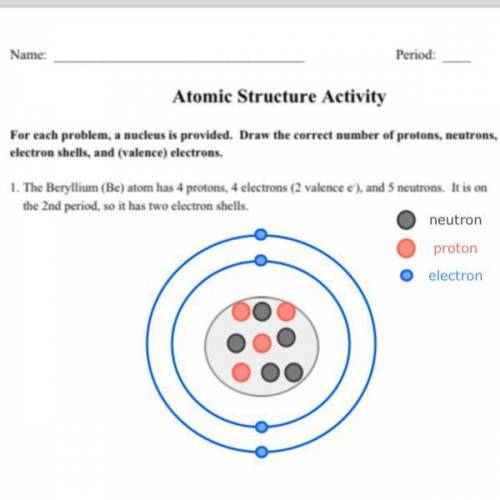
The Beryllium (Be) atom has 4 protons, 4 electrons (2 valence e- ), and 5 neutrons. It is on the 2...
Questions

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Business, 16.11.2020 14:00

Business, 16.11.2020 14:00

English, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

English, 16.11.2020 14:00

Chemistry, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00


English, 16.11.2020 14:00

Mathematics, 16.11.2020 14:00