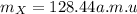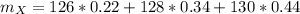Chemistry, 09.02.2021 07:50 murphyscott794
An unknown element X has the following isotopes: 126X (22.00%
abundant), 128X (34.00% abundant), 130X (44.00% abundant). What is
the average atomic mass in amu of X?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
An unknown element X has the following isotopes: 126X (22.00%
abundant), 128X (34.00% abundant), 13...
Questions


Health, 06.06.2021 03:20

Mathematics, 06.06.2021 03:20

Computers and Technology, 06.06.2021 03:20




Mathematics, 06.06.2021 03:20


Mathematics, 06.06.2021 03:30



English, 06.06.2021 03:30




Advanced Placement (AP), 06.06.2021 03:30