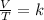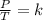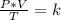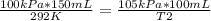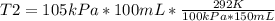Chemistry, 09.02.2021 04:30 ashtonbillups
150.0 mL of oxygen is collected over water at 19.0oC and 100.0 kPa. If the dry volume becomes 100.0 mL and the pressure of the dry gas becomes 105.0 kPa, what will the new temperature be?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

You know the right answer?
150.0 mL of oxygen is collected over water at 19.0oC and 100.0 kPa. If the dry volume becomes 100.0...
Questions

Mathematics, 03.02.2021 21:30

History, 03.02.2021 21:30



Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30


Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30


Chemistry, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30



Mathematics, 03.02.2021 21:30




Mathematics, 03.02.2021 21:30