Chemistry, 09.02.2021 04:10 casting479
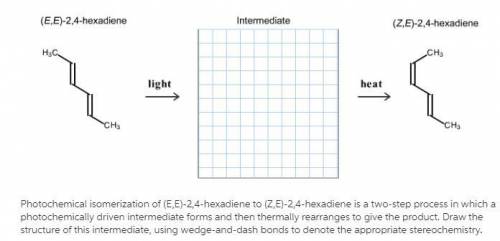
Photochemical isomerization of (E, E)-2,4-hexadiene to (Z, E)-2,4-hexadiene is a two-step process in which a photochemically driven intermediate forms and then thermally rearranges to give the product. Draw the structure of this intermediate, using wedge-and-dash bonds to denote the appropriate stereochemistry.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

You know the right answer?
Photochemical isomerization of (E, E)-2,4-hexadiene to (Z, E)-2,4-hexadiene is a two-step process in...
Questions


History, 23.10.2020 15:20

Mathematics, 23.10.2020 15:20

Mathematics, 23.10.2020 15:20



Mathematics, 23.10.2020 15:20



Computers and Technology, 23.10.2020 15:20

Mathematics, 23.10.2020 15:20

English, 23.10.2020 15:20

History, 23.10.2020 15:20



Mathematics, 23.10.2020 15:20


Chemistry, 23.10.2020 15:20