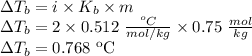Chemistry, 09.02.2021 03:50 vincentfriend
What is the boiling point of a solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
What is the boiling point of a solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water?...
Questions


History, 18.09.2021 05:40


English, 18.09.2021 05:40


Physics, 18.09.2021 05:40

Mathematics, 18.09.2021 05:40

Chemistry, 18.09.2021 05:40

Chemistry, 18.09.2021 05:40



Biology, 18.09.2021 05:40


Social Studies, 18.09.2021 05:40

English, 18.09.2021 05:40

Mathematics, 18.09.2021 05:40

Mathematics, 18.09.2021 05:40

Advanced Placement (AP), 18.09.2021 05:40

History, 18.09.2021 05:40

Mathematics, 18.09.2021 05:40

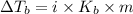
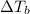 is the change in the water's boiling point (normally taken to be 100 °C),
is the change in the water's boiling point (normally taken to be 100 °C),  is the Van 't Hoff factor (the number of particles a single formula unit of the solute dissociates into in water),
is the Van 't Hoff factor (the number of particles a single formula unit of the solute dissociates into in water), 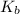 is the boiling point elevation constant, and
is the boiling point elevation constant, and 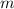 is the molality (moles of solute/kilogram(s) of solvent) of the solution.
is the molality (moles of solute/kilogram(s) of solvent) of the solution.