
Chemistry, 09.02.2021 01:50 courtney3652
The equilibrium constant, Kc, for the following reaction is 83.3 at 500 K. PCl3(g) Cl2(g) PCl5(g) Calculate the equilibrium concentrations of reactant and products when 0.453 moles of PCl3 and 0.453 moles of Cl2 are introduced into a 1.00 L vessel at 500 K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

You know the right answer?
The equilibrium constant, Kc, for the following reaction is 83.3 at 500 K. PCl3(g) Cl2(g) PCl5(g) Ca...
Questions

Mathematics, 11.02.2021 21:20


Mathematics, 11.02.2021 21:20

Mathematics, 11.02.2021 21:20

Mathematics, 11.02.2021 21:20

Mathematics, 11.02.2021 21:20

Social Studies, 11.02.2021 21:20



Mathematics, 11.02.2021 21:20


Mathematics, 11.02.2021 21:20

English, 11.02.2021 21:20

Biology, 11.02.2021 21:20

Mathematics, 11.02.2021 21:20

Arts, 11.02.2021 21:20




![[PCl_3]=[Cl_2]=0.068M](/tpl/images/1103/3296/1ad55.png)
![[PCl_5]=0.385M](/tpl/images/1103/3296/9d9e6.png)
![Kc=\frac{[PCl_5]}{[Cl_2][PCl_3]}](/tpl/images/1103/3296/6b2bc.png)
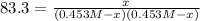
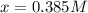
![[PCl_3]=[Cl_2]=0.453M-0.385M=0.068M](/tpl/images/1103/3296/82c25.png)



