
Chemistry, 09.02.2021 01:00 Josediego55
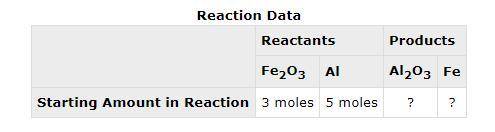
The following data was collected when a reaction was performed experimentally in the laboratory. Determine the maximum amount of Fe that was produced during the experiment. Explain how you determined this amount.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
The following data was collected when a reaction was performed experimentally in the laboratory.
De...
Questions


Mathematics, 21.07.2019 08:30


Mathematics, 21.07.2019 08:30

Mathematics, 21.07.2019 08:30


Mathematics, 21.07.2019 08:30


Mathematics, 21.07.2019 08:30

Social Studies, 21.07.2019 08:30

History, 21.07.2019 08:30



Mathematics, 21.07.2019 08:30





Mathematics, 21.07.2019 08:30

Mathematics, 21.07.2019 08:30



