
Chemistry, 09.02.2021 01:00 eburnhisel2023
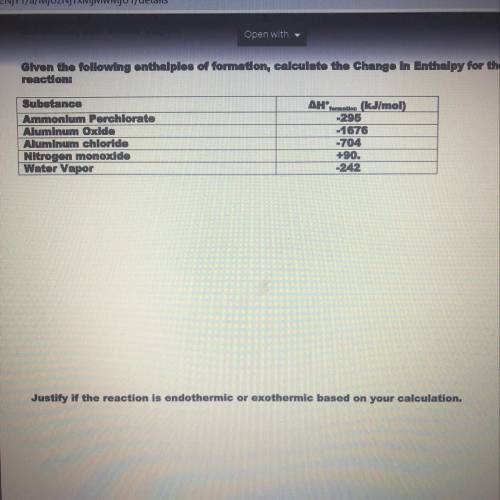
Given the following enthalpies of formation calculate the change in enthalpy for the reaction. Then justify if the reaction is endothermic or exothermic based on your calculations. PLEASE HELP WILL GIVE BRAINLIEST

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Given the following enthalpies of formation calculate the change in enthalpy for the reaction. Then...
Questions


Mathematics, 07.01.2021 19:10

Mathematics, 07.01.2021 19:10



Mathematics, 07.01.2021 19:10

History, 07.01.2021 19:10





Mathematics, 07.01.2021 19:10

Mathematics, 07.01.2021 19:10

Mathematics, 07.01.2021 19:10

Mathematics, 07.01.2021 19:10




Biology, 07.01.2021 19:10



