
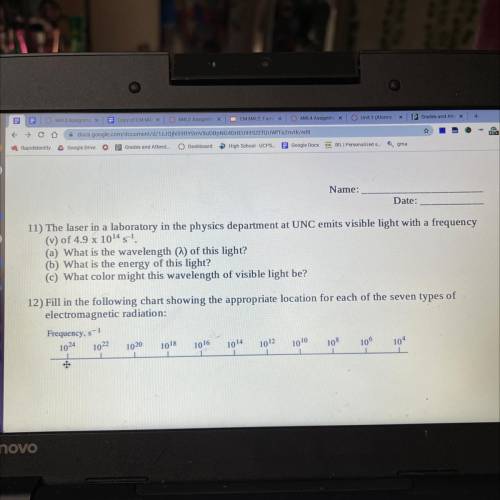
11) The laser in a laboratory in the physics department at UNC emits visible light with a frequency
(v) of 4.9 x 1014 s-1.
(a) What is the wavelength (m) of this light?
(b) What is the energy of this light?
(C) What color might this wavelength of visible light be?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

You know the right answer?
11) The laser in a laboratory in the physics department at UNC emits visible light with a frequency...
Questions




Advanced Placement (AP), 10.03.2021 19:30

English, 10.03.2021 19:30



Mathematics, 10.03.2021 19:30

Advanced Placement (AP), 10.03.2021 19:30

Computers and Technology, 10.03.2021 19:30


Mathematics, 10.03.2021 19:30

Chemistry, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30


Chemistry, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30

English, 10.03.2021 19:30

Mathematics, 10.03.2021 19:30



