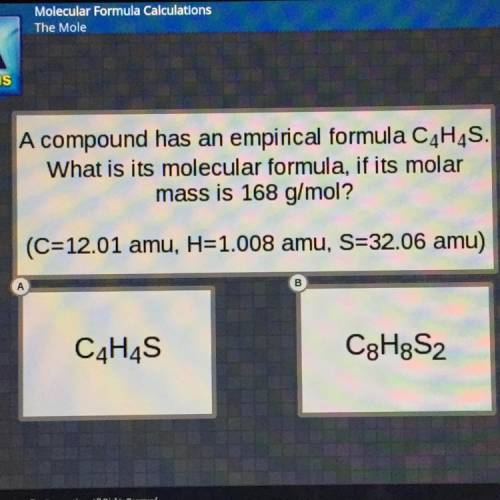
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass i...

Chemistry, 09.02.2021 01:00 natalyarenassalgado
A compound has an empirical formula C4H4S.
What is its molecular formula, if its molar
mass is 168 g/mol?
(C=12.01 amu, H=1.008 amu, S=32.06 amu)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
Questions



Geography, 05.03.2020 13:31



English, 05.03.2020 13:31


Mathematics, 05.03.2020 13:31

Mathematics, 05.03.2020 13:32

Computers and Technology, 05.03.2020 13:32

Computers and Technology, 05.03.2020 13:33

English, 05.03.2020 13:33










