Chemistry, 08.02.2021 23:40 wittlerylee34
Naturally occurring silicon consists of three isotopes with the following isotopic masses and abundances. IsotopeIsotopic mass (u)Abundance (%) 28 Si 27.9769265327 92.2297 29 Si 28.97649472 4.6832 30 Si 29.97377022 3.0872 Calculate the average atomic mass of naturally occurring silicon to at least four significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Naturally occurring silicon consists of three isotopes with the following isotopic masses and abunda...
Questions

Health, 11.10.2019 00:00


Geography, 11.10.2019 00:00



Social Studies, 11.10.2019 00:00

Biology, 11.10.2019 00:00


Physics, 11.10.2019 00:00

Chemistry, 11.10.2019 00:00


Chemistry, 11.10.2019 00:00


History, 11.10.2019 00:00


Social Studies, 11.10.2019 00:00


Physics, 11.10.2019 00:00


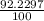
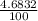
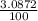
![A=\sum[(27.9769265327\times \frac{92.2297}{100})+(28.97649472\times \frac{4.6832}{100})+(29.97377022\times \frac{3.0872}{100})]](/tpl/images/1102/8397/1dfea.png)