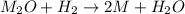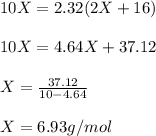Chemistry, 08.02.2021 14:00 Nickanderson21
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal
and one mole of water. 5.00 g of the metallic oxide produces 2.32 g of the metal. What is the metallic
oxide? (Use molar masses)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal...
Questions

Mathematics, 26.01.2021 02:00


Mathematics, 26.01.2021 02:00



Mathematics, 26.01.2021 02:00


Mathematics, 26.01.2021 02:00



Mathematics, 26.01.2021 02:00


English, 26.01.2021 02:00





History, 26.01.2021 02:00

English, 26.01.2021 02:00

Mathematics, 26.01.2021 02:00