
ANSWER ASAP
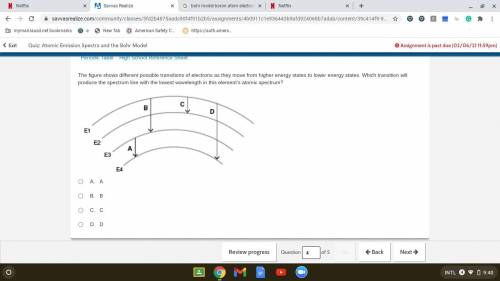
The figure shows different possible transitions of electrons as they move from higher energy states to lower energy states. Which transition will produce the spectrum line with the lowest wavelength in this element’s atomic spectrum?
A. A
B. B
C. C
D. D

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
ANSWER ASAP
The figure shows different possible transitions of electrons as they move from higher e...
Questions


Biology, 05.11.2019 13:31


Physics, 05.11.2019 13:31



Mathematics, 05.11.2019 13:31

Mathematics, 05.11.2019 13:31

Biology, 05.11.2019 13:31


Social Studies, 05.11.2019 13:31

History, 05.11.2019 13:31

History, 05.11.2019 13:31


Social Studies, 05.11.2019 13:31


Mathematics, 05.11.2019 13:31

History, 05.11.2019 13:31

English, 05.11.2019 13:31

Biology, 05.11.2019 13:31




