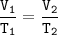Chemistry, 06.02.2021 07:50 jaimejohnston2
An arctic weather balloon is filled with 4.96 L of helium gas inside a prep shed. The temperature inside the shed is 6. °C. The balloon is then taken outside, where the temperature is 2. °C. Calculate the new volume of the balloon.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
An arctic weather balloon is filled with 4.96 L of helium gas inside a prep shed. The temperature in...
Questions

Mathematics, 04.02.2021 19:40


Mathematics, 04.02.2021 19:40

Mathematics, 04.02.2021 19:40

English, 04.02.2021 19:40

Mathematics, 04.02.2021 19:40


Mathematics, 04.02.2021 19:40


Mathematics, 04.02.2021 19:40

English, 04.02.2021 19:40


Mathematics, 04.02.2021 19:40



English, 04.02.2021 19:40

English, 04.02.2021 19:40

History, 04.02.2021 19:40

Mathematics, 04.02.2021 19:40

Mathematics, 04.02.2021 19:40