Chemistry, 06.02.2021 02:00 sarah19Nursing
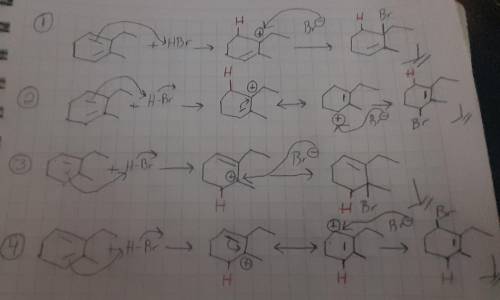
Draw all four products obtained when 2-ethyl-3-methyl-1,3-cyclohexadiene is treated with HBr at room temperature and show the mechanism of their formation. For the mechanism, include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly. Do not use abbreviations such as Me or Ph.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Draw all four products obtained when 2-ethyl-3-methyl-1,3-cyclohexadiene is treated with HBr at room...
Questions

Physics, 01.10.2019 08:30


Mathematics, 01.10.2019 08:30

Advanced Placement (AP), 01.10.2019 08:30




English, 01.10.2019 08:30

Computers and Technology, 01.10.2019 08:30




Mathematics, 01.10.2019 08:30




Mathematics, 01.10.2019 08:30


Mathematics, 01.10.2019 08:30

Mathematics, 01.10.2019 08:30