
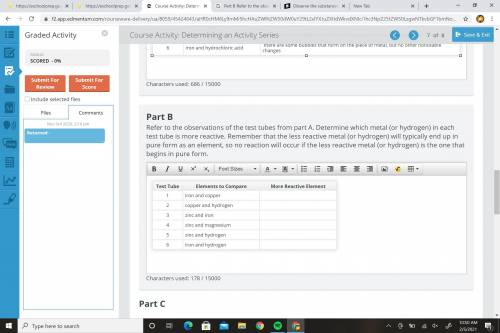
Please help me... Refer to the observations of the test tubes from part A. Determine which metal (or hydrogen) in each test tube is more reactive. Remember that the less reactive metal (or hydrogen) will typically end up in pure form as an element, so no reaction will occur if the less reactive metal (or hydrogen) is the one that begins in pure form.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:02
If 0.414 g of hydrogen is obtained in this experiment how many grams of sulfur must be obtained
Answers: 2

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

You know the right answer?
Please help me...
Refer to the observations of the test tubes from part A. Determine which metal (...
Questions






Spanish, 28.07.2019 02:30

Biology, 28.07.2019 02:30



Advanced Placement (AP), 28.07.2019 02:30


Geography, 28.07.2019 02:30

History, 28.07.2019 02:30





History, 28.07.2019 02:30





