
Consider this reaction: 2so2 (g) + o2 (g) 2so3 (g) + heat. what will happen if more sulfur trioxide gas is added to the reaction mixture?
a-the equilibrium will shift toward the products.
b-the equilibrium will shift toward the reactants.
c-keq will increase.
d-keq will decrease.
e-there will be no shift in equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1



Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Consider this reaction: 2so2 (g) + o2 (g) 2so3 (g) + heat. what will happen if more sulfur trioxide...
Questions





Mathematics, 28.11.2019 22:31

History, 28.11.2019 22:31

Mathematics, 28.11.2019 22:31

English, 28.11.2019 22:31

History, 28.11.2019 22:31

History, 28.11.2019 22:31

Social Studies, 28.11.2019 22:31



Geography, 28.11.2019 22:31

Chemistry, 28.11.2019 22:31

Mathematics, 28.11.2019 22:31



Mathematics, 28.11.2019 22:31

Mathematics, 28.11.2019 22:31

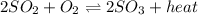
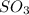 is added
is added

