
Chemistry, 05.02.2021 03:30 b2cutie456
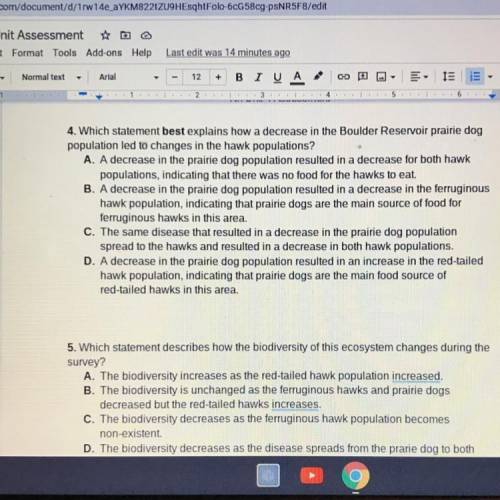
4. Which statement best explains how a decrease in the Boulder Reservoir prairie dog
population led to changes in the hawk populations?
A. A decrease in the prairie dog population resulted in a decrease for both hawk
populations, indicating that there was no food for the hawks to eat.
B. A decrease in the prairie dog population resulted in a decrease in the ferruginous
hawk population, indicating that prairie dogs are the main source of food for
ferruginous hawks in this area.
C. The same disease that resulted in a decrease in the prairie dog population
spread to the hawks and resulted in a decrease in both hawk populations.
D. A decrease in the prairie dog population resulted in an increase in the red-tailed
hawk population, indicating that prairie dogs are the main food source of
red-tailed hawks in this area.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
4. Which statement best explains how a decrease in the Boulder Reservoir prairie dog
population led...
Questions


Mathematics, 16.02.2021 09:10

Mathematics, 16.02.2021 09:10

Chemistry, 16.02.2021 09:10


Chemistry, 16.02.2021 09:10

Biology, 16.02.2021 09:10

Computers and Technology, 16.02.2021 09:10


English, 16.02.2021 09:10

Mathematics, 16.02.2021 09:10


English, 16.02.2021 09:10

Mathematics, 16.02.2021 09:10




Mathematics, 16.02.2021 09:10

History, 16.02.2021 09:10

Mathematics, 16.02.2021 09:10



