
Chemistry, 04.02.2021 21:10 jonquil201
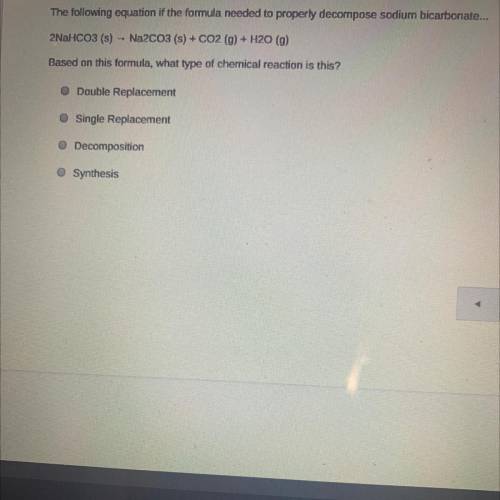
The following equation if the formula needed to properly decompose sodium bicarbonate...
2NaHCO3(s)
Na2CO3 (s) + CO2 (g) + H2O (9)
Based on this formula, what type of chemical reaction is this?
O Double Replacement
O Single Replacement
Decomposition
Synthesis
1
1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
The following equation if the formula needed to properly decompose sodium bicarbonate...
2NaHCO3(s)...
Questions

Mathematics, 30.12.2020 07:10


Mathematics, 30.12.2020 07:10




English, 30.12.2020 07:10

English, 30.12.2020 07:10

Engineering, 30.12.2020 07:10

Mathematics, 30.12.2020 07:10

Health, 30.12.2020 07:10


Mathematics, 30.12.2020 07:10

World Languages, 30.12.2020 07:10

Mathematics, 30.12.2020 07:10


Computers and Technology, 30.12.2020 07:10



Chemistry, 30.12.2020 07:10



