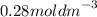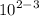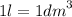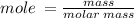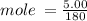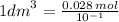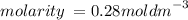Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
Calculate the molarity of a solution made by dissolving 5.00 g of glucose (C6H12O6) in sufficient wa...
Questions

Social Studies, 26.02.2021 23:40


Mathematics, 26.02.2021 23:40


Mathematics, 26.02.2021 23:40


History, 26.02.2021 23:40


Mathematics, 26.02.2021 23:40


Mathematics, 26.02.2021 23:40



Mathematics, 26.02.2021 23:40

Mathematics, 26.02.2021 23:40

Advanced Placement (AP), 26.02.2021 23:40

Mathematics, 26.02.2021 23:40


Social Studies, 26.02.2021 23:40

Mathematics, 26.02.2021 23:40