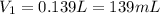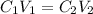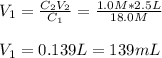Chemistry, 03.02.2021 04:10 deasiamonay14
concentrated sulfuric acid (H2SO4) has a concentration of 18.0 M. What volume of concentrated H2SO4 is needed to make 2.5 liters of a 1.0 M solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

You know the right answer?
concentrated sulfuric acid (H2SO4) has a concentration of 18.0 M. What volume of concentrated H2SO4...
Questions

Mathematics, 02.12.2020 21:30


History, 02.12.2020 21:30


Business, 02.12.2020 21:30

Biology, 02.12.2020 21:30

Arts, 02.12.2020 21:30


Business, 02.12.2020 21:30


Mathematics, 02.12.2020 21:30


Mathematics, 02.12.2020 21:30

Chemistry, 02.12.2020 21:30

Mathematics, 02.12.2020 21:30