Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3


Chemistry, 23.06.2019 13:30
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
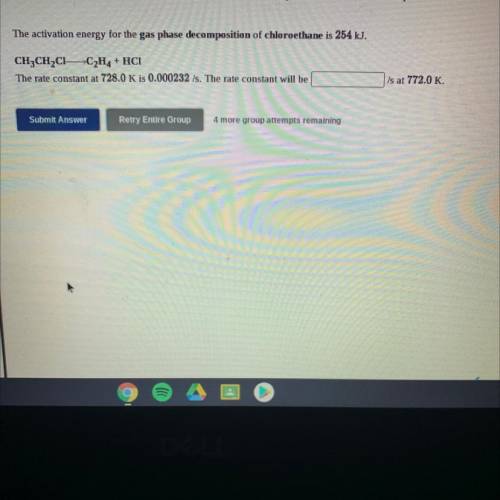
The activation energy for the gas phase decomposition of chloroethane is 254 kJ.
CH3CH2C1 -C2H4 + H...
Questions



History, 27.02.2020 05:44






Mathematics, 27.02.2020 05:45


Mathematics, 27.02.2020 05:45





English, 27.02.2020 05:45

Mathematics, 27.02.2020 05:45

Mathematics, 27.02.2020 05:46

Mathematics, 27.02.2020 05:46