Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
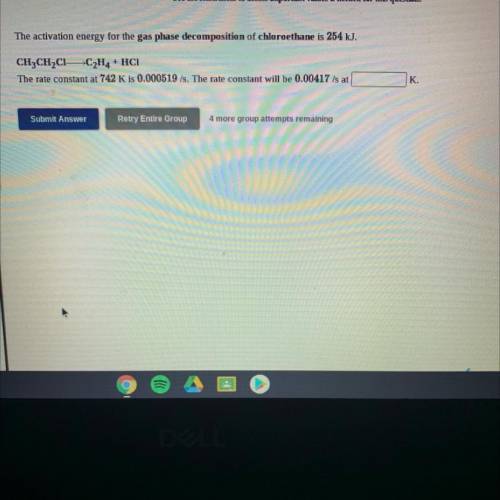
The activation energy for the gas phase decomposition of chloroethane is 254 kJ.
CH3CH2C1-C2H4 + HC...
Questions

History, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01

History, 16.10.2020 18:01

Biology, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Advanced Placement (AP), 16.10.2020 18:01




History, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

English, 16.10.2020 18:01

Social Studies, 16.10.2020 18:01