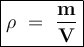Fish in a frozen lake can survive throughout the winter months due
partially to the fact that lakes don't freeze solid. Fresh water has a density
of 1.00 g/cm3. A piece of ice has a mass of 57g and a volume of 62 cm3.
Give the density of the piece of ice (use the density formula d = m/V) and
explain what will happen to it in the lake.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Fish in a frozen lake can survive throughout the winter months due
partially to the fact that lakes...
Questions



Arts, 02.11.2020 19:50

English, 02.11.2020 19:50


Mathematics, 02.11.2020 19:50





History, 02.11.2020 19:50


English, 02.11.2020 19:50


Mathematics, 02.11.2020 19:50



English, 02.11.2020 19:50