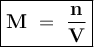Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

You know the right answer?
The molecular weight of KMnO4 is 158 g/mol. If you wanted to make 500 mL of a 0.1M stock solution of...
Questions

Biology, 22.11.2019 00:31







Mathematics, 22.11.2019 00:31

Mathematics, 22.11.2019 00:31


Social Studies, 22.11.2019 00:31

Health, 22.11.2019 00:31

Mathematics, 22.11.2019 00:31

Mathematics, 22.11.2019 00:31

History, 22.11.2019 00:31


Physics, 22.11.2019 00:31


Chemistry, 22.11.2019 00:31