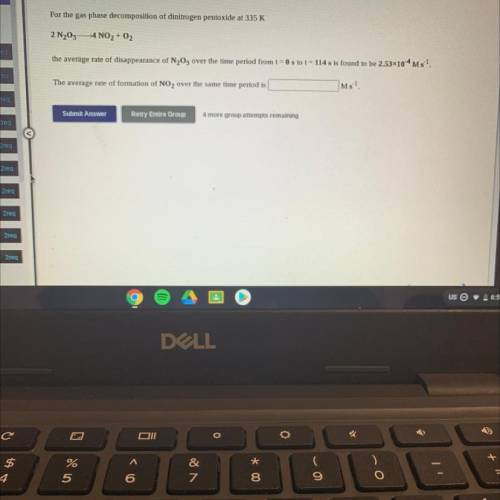
For the gas phase decomposition of dinitrogen pentoxide at 335 K
2 N205– 4 NO2 + O2
the avera...

For the gas phase decomposition of dinitrogen pentoxide at 335 K
2 N205– 4 NO2 + O2
the average rate of disappearance of N205 over the time period from t = 0 s to t = 114 s is found to be 2.53x10-4 M 5-1.
The average rate of formation of NO2 over the same time period is
Ms-1
Submit Answer
Retry Entire Group
4 more group attempts remaining

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2


Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Questions


Computers and Technology, 27.09.2019 09:50




English, 27.09.2019 09:50

Chemistry, 27.09.2019 09:50


Geography, 27.09.2019 09:50






Social Studies, 27.09.2019 09:50

Mathematics, 27.09.2019 09:50


Mathematics, 27.09.2019 09:50


History, 27.09.2019 09:50



