
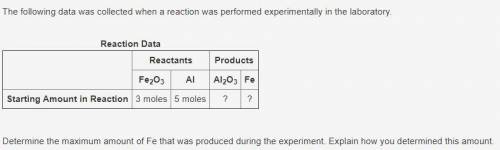
PLEASE HELP!! The following data was collected when a reaction was performed experimentally in the laboratory.
Reaction Data:
Reactants:
Fe2O3 (3 moles)
Al (5 moles)
Products:
Al2O3 (?)
Fe (?)
Determine the maximum amount of Fe that was produced during the experiment. Explain how you determined this amount.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
PLEASE HELP!! The following data was collected when a reaction was performed experimentally in the l...
Questions

Mathematics, 31.01.2021 09:30



Advanced Placement (AP), 31.01.2021 09:30



English, 31.01.2021 09:30


Mathematics, 31.01.2021 09:30


English, 31.01.2021 09:30





Mathematics, 31.01.2021 09:30

Mathematics, 31.01.2021 09:30

Mathematics, 31.01.2021 09:30


Mathematics, 31.01.2021 09:30



