

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
1,200 cal of heat energy is added to a liquid with a specific heat of 0.57 cal/g°C. If the temperatu...
Questions

Physics, 04.04.2020 18:02

Biology, 04.04.2020 18:02

Mathematics, 04.04.2020 18:02

Mathematics, 04.04.2020 18:02

English, 04.04.2020 18:02


English, 04.04.2020 18:02

Mathematics, 04.04.2020 18:02


Health, 04.04.2020 18:03


Mathematics, 04.04.2020 18:03


Business, 04.04.2020 18:03


Mathematics, 04.04.2020 18:04

Mathematics, 04.04.2020 18:04

Mathematics, 04.04.2020 18:04


Chemistry, 04.04.2020 18:04

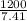 = 161.943 ≈ 162 g
= 161.943 ≈ 162 g

