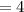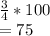Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1


Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
What is the percent/% yield if 4 moles of hydrogen is reacted with 3 moles
of oxygen and produces 3...
Questions



English, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30


Social Studies, 18.03.2021 01:30


Chemistry, 18.03.2021 01:30




English, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Chemistry, 18.03.2021 01:30

Chemistry, 18.03.2021 01:30

Physics, 18.03.2021 01:30

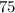 %
%