
Chemistry, 30.01.2021 16:10 kaylee0424
PLEASE HELP ME
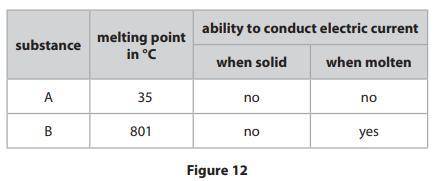
Figure 12 shows the melting points of two substances, A and B, and the abilities
of the substances to conduct an electric current when solid and when molten.
One of the substances has an ionic structure and one has a simple molecular,
covalent structure.
Explain, in terms of bonding and the forces between the particles, the relative
melting points and abilities to conduct the electric current of substances A and B.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
PLEASE HELP ME
Figure 12 shows the melting points of two substances, A and B, and the abilities
Questions

Chemistry, 04.02.2021 09:30


Advanced Placement (AP), 04.02.2021 09:30




English, 04.02.2021 09:30




Mathematics, 04.02.2021 09:30

Mathematics, 04.02.2021 09:30



Geography, 04.02.2021 09:30


Mathematics, 04.02.2021 09:30


English, 04.02.2021 09:30

English, 04.02.2021 09:30



