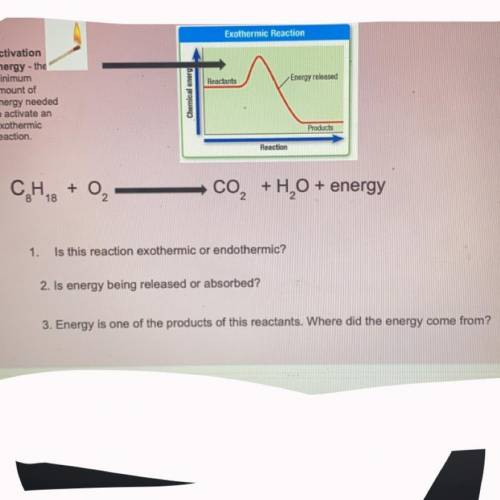
Help me with this please I need the answers ASAP
...

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Questions


Mathematics, 29.06.2019 00:00

English, 29.06.2019 00:00

Biology, 29.06.2019 00:00

Physics, 29.06.2019 00:00



Mathematics, 29.06.2019 00:10






Computers and Technology, 29.06.2019 00:10






Computers and Technology, 29.06.2019 00:10