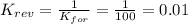I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure would
N2(g)+3H2(g)⇄2NH3(g)
a. increase [H2]
b. increase [NH3]
c. increase [N2]
d. have no effect
2. If K=100, then the value of K for the reverse reaction is
a. the same value
b. can only be determined by experimentation
c. the negative of the value for the forward reaction
d. 0.01

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure woul...
Questions



History, 17.07.2019 09:50



Mathematics, 17.07.2019 09:50


Mathematics, 17.07.2019 09:50


Mathematics, 17.07.2019 09:50




History, 17.07.2019 09:50

English, 17.07.2019 09:50




Mathematics, 17.07.2019 09:50

English, 17.07.2019 09:50