
Chemistry, 30.01.2021 06:10 iamabouttofail
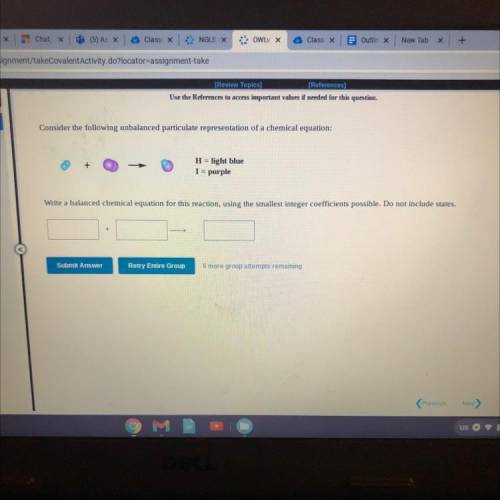
Consider the following unbalanced particulate representation of a chemical equation:
H = light blue
I = purple
Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1


Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
Consider the following unbalanced particulate representation of a chemical equation:
H = light blue...
Questions



English, 06.09.2021 21:30

Biology, 06.09.2021 21:30

Mathematics, 06.09.2021 21:30





English, 06.09.2021 21:30




Mathematics, 06.09.2021 21:30

Medicine, 06.09.2021 21:30


Computers and Technology, 06.09.2021 21:30


English, 06.09.2021 21:30



