
Chemistry, 30.01.2021 05:40 dominguezjose625
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-6 in orange and black. The black numbers
are the wavelengths in nanometers of the associated electromagnetic radiation.
Which wavelength is associated with a photon energy of 1.13 electron volts?
Bohr Model of the Hydrogen Atom
ultraviolet
infrared
-97
103
1,094
122
1,282
1,875
visible
410
434
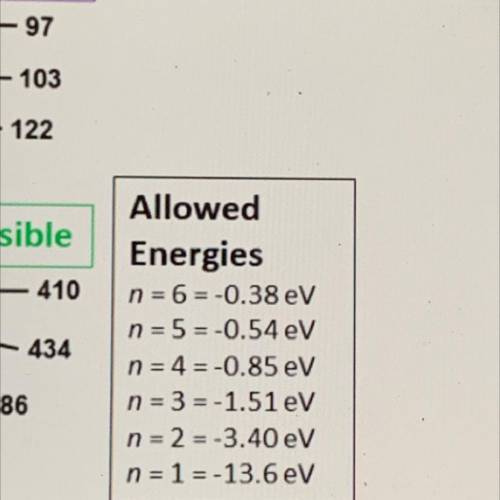
Allowed
Energies
n = 6-0.38 eV
n = 5=-0.54 eV
n = 4 = -0.85 eV
n = 3 = -1.51 eV
n = 2 = -3.40 eV
n = 1 = -13.6 eV
486
656
In orbits
n orbits

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1


Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2

Chemistry, 23.06.2019 13:20
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2
You know the right answer?
An illustration of the Bohr model of the hydrogen atom is shown. The electron orbits are numbered 1-...
Questions


History, 01.07.2019 01:50

English, 01.07.2019 01:50

Mathematics, 01.07.2019 01:50

Mathematics, 01.07.2019 01:50

Mathematics, 01.07.2019 01:50


Biology, 01.07.2019 01:50

English, 01.07.2019 01:50

Mathematics, 01.07.2019 01:50

English, 01.07.2019 01:50


Mathematics, 01.07.2019 01:50


History, 01.07.2019 01:50

English, 01.07.2019 01:50


Health, 01.07.2019 01:50




