
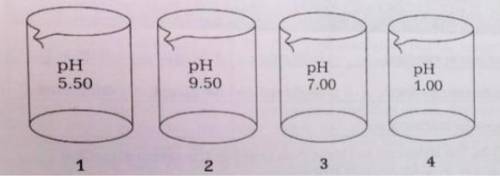
The four beakers above each contain 100.-mL of different solutions of similar concentrations.
(a) The Kb for ammonia is 1.8 x 10^-5
(i) Which beaker is most likely to contain NH3(aq)? Provide a chemical equation to explain your answer.
(ii) Calculate the molarity of the solution in the beaker that you chose for (i).
(b) If the contents of beakers 3 and 4 are poured together and mixed thoroughly, what will be the resulting pH?
(c) Explain how it is possible that beakers 1 and 4 are acids with equal molarities.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

You know the right answer?
The four beakers above each contain 100.-mL of different solutions of similar concentrations.
(a) T...
Questions

Social Studies, 12.01.2021 01:00

English, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00


History, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00





Mathematics, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

Physics, 12.01.2021 01:00


Business, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00


Health, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00



